Plateco, Inc. Case Study: To Passivate or Not to Passivate After Hydrogen Embrittlement Relief
In our last Plateco Pointer, “Hydrogen Embrittlement: What It Is and How to Combat It,” we discussed how hydrogen embrittlement occurs, its consequences, and the hydrogen embrittlement relief baking process. While baking product does remove hydrogen and reinstate the strength of steel, it also has a negative effect on the passivate topcoat, causing the passivate to dehydrate and lead to premature white corrosion.
In zinc plating, there are three possible layers applied to steel to achieve a desired corrosion resistance. The first layer is zinc, the second layer is a hexavalent chromate or trivalent passivate, and the third is a wax or sealer. Both the second and third layers are optional depending on the specification and desired corrosion resistance.
Plateco Pointer:
Understanding the Difference Between Chromates and Passivates
A passivate is a chemical compound whose purpose is to protect the zinc layer; as zinc protects steel substrate from red corrosion, passivates are essential to guard zinc from white corrosion. While white corrosion may seem like the product failing since it is zinc that is corroding, in the eyes of a zinc plater, this is actually a good thing; it means the zinc is doing its job and sacrificing itself for the base material. Without a passivate though, there is nothing protecting the zinc and this white corrosion could begin as soon as 24 hours after plating. Passivates typically measure from 0.6 to 1 micron thick depending on if it is a thin film or a thick film. Thin film passivates can provide 96-120 hours of protection in a salt spray chamber depending on color and quality, whereas thick film passivates can provide 168-240 hours depending on color and quality. Actual results may vary depending on how the application process is used (rack plating vs. barrel plating).
The Effect of Baking on Passivates
At Plateco, when we bake product, we process on three separate production lines. The first line is to add the zinc plating and the passivate. The second line is to bake the product for hydrogen embrittlement relief. During the baking process, product is subjected to immense heat and as passivates are most often the outermost layer, they absorb the full force of this heat. This causes passivates to become dehydrated and lessens their protection capability. As the baking process compromises the integrity of passivate coatings, the third and last production line is to reapply the passivate.
Many platers and customers do choose to forego reapplying passivates after hydrogen embrittlement relief due to the cost. However, many are not aware that most product no longer meets corrosion resistance requirements without reapplying this passivate.
Testing: Passivate After Baking Vs. No Passivate After Baking
In Sept. 2021, Plateco conducted a test to determine the corrosion resistance of zinc plated steel that received a passivate reapplication after baking versus one that did not. Below are images that show two plated test panels side by side and their progression through corrosion in a salt spray chamber. Plateco plated both test panels directly beside each other in the same rack plating process with .0005” average of zinc.
To ensure the consistency and quality of the test results, the salt spray chamber was opened once a day at 2:00 pm for approximately two minutes. The purpose of opening the chamber was for record keeping and image taking. In the images below, the term “Add Too” refers to the process of reapplying passivates.
Enter Salt Spray Chamber
No reapplication: 0% white corrosion
Passivate reapplication: 0% white corrosion
24 Hours in Salt Spray Chamber
No reapplication: 70% white corrosion
Passivate reapplication: 0% white corrosion
96 Hours in Salt Spray Chamber
No reapplication: 100% white corrosion
Passivate reapplication: 0% white corrosion
168 Hours in Salt Spray Chamber
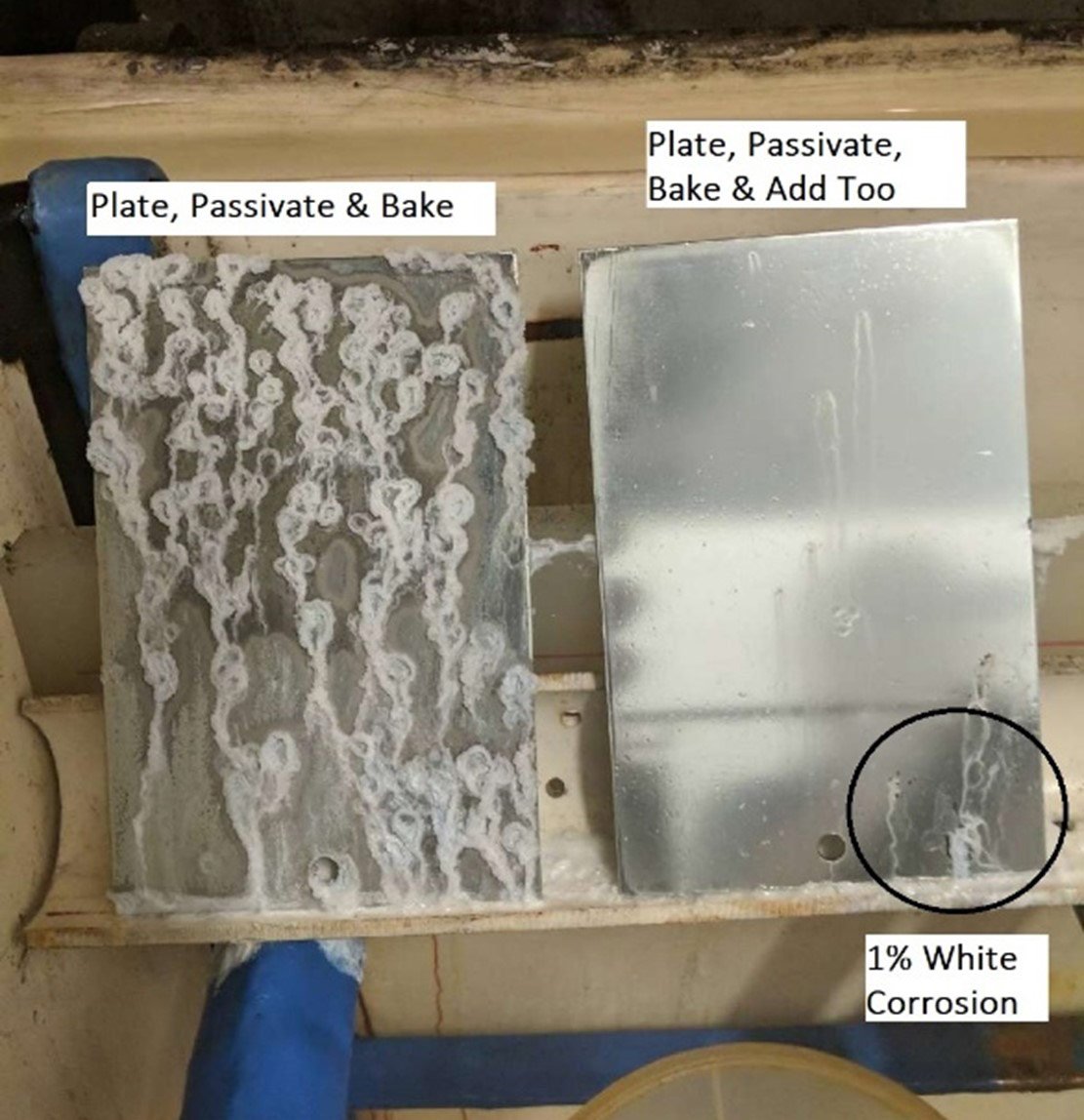
No reapplication: 100% white corrosion
Passivate reapplication: 1% white corrosion
The results of our testing are fairly conclusive; the sample panel that received another coating of passivate after baking for hydrogen embrittlement relief performed significantly better during salt spray testing than the panel that did not receive a second passivate coating. At 24 hours of testing, the panel that did not receive a second application contained 1% white corrosion while the panel that did receive the second application had no signs of white corrosion. At 168 hours, the panel without the second application had been at 100% white corrosion for at least 72 hours while the panel that did receive the second application had reached 1%. As is exhibited through this experiment, steel product that receives a reapplication of a passivate topcoat after hydrogen embrittlement relief yields better corrosion resistance.
Depending on the end use of the part, the specification, and the customer, the option of whether or not to reapply the passivate essentially comes down to the age-old debate, Price Vs. Cost. Is a cheaper price worth the sacrifice in corrosion protection? Or is higher quality more important than a higher price?